Research
Protein is the key player of tremendous life activities. Our research group has great interests in natural or artificial modifications on protein sequence.
Research Overview
Protein is the key player of tremendous life activities, which are achieved through the interaction network of protein with different biomolecules. Our research group have great interests in natural or artificial modifications on protein sequence composed of canonical amino acids, which endow it with largely expanded properties and function. Both mechanistic elucidation of natural modifications' regulation and modulation of protein-biomolecules interaction for biomedical purpose urgently require structure-defined homogeneous modified proteins and peptides. We mainly focus on three research topics describes as follows.
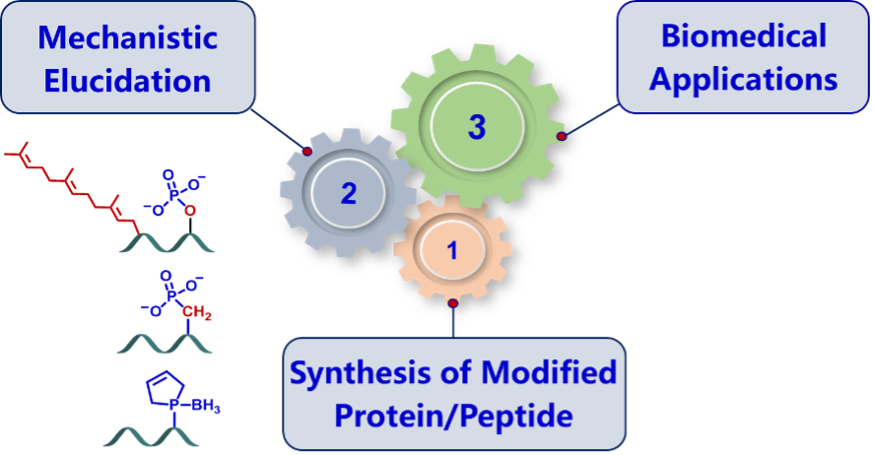
I. Synthesis of Modified Proteins for Mechanistic Investigation
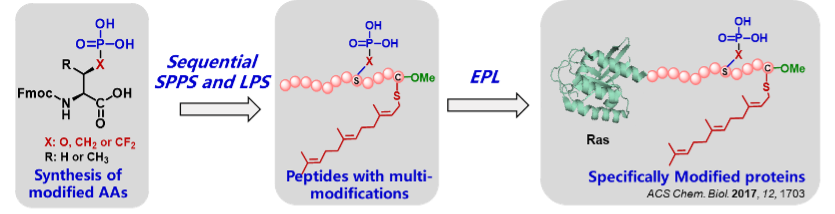
Both hydrophobic farnesyl moiety and charged phosphate in the flexible region of key signaling proteins, like K-Ras and Rnd3, regulate their cellular distribution and activity, but the molecular mechanism remains debatable and unsolved mainly due to the difficulty of obtaining homogeneous proteins with multi-modifications.
We have intended to develop efficient synthetic strategies for accessing to pathological modified proteins by combining chemical and biological synthesis techniques, like K-Ras and Rnd3 simultaneously embodying phosphate, farnesyl moiety and C-terminal methyl ester, which are applied to uncover the regulation mechanism of various modifications on proteins' interaction network.
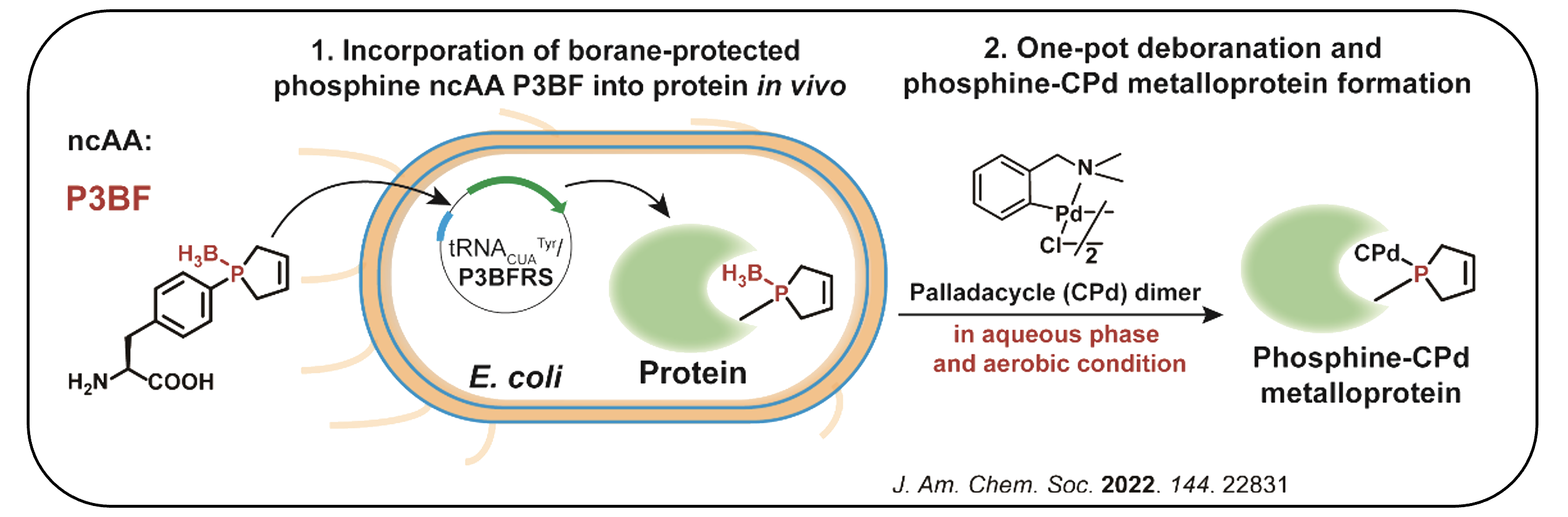
II. Synthesis of Protein Phosphine Ligand for Metalloprotein Design
Phosphorus at low oxidation state, as the privileged ligand of many renowned transitional metal catalysts, never appears in living organisms to function probably due to its oxygen sensitivity, while natural metalloproteins usually rely on nitrogen, sulfur and oxygen ligands.
We have focused on developing bio-synthetic tools for accessing these specifically phosphine-modified proteins, which must overcome the oxygen sensitivity of phosphine, as well as realizing transformation into metalloproteins via phosphine ligand. This platform can enable the rational design of novel artificial metalloenzymes.
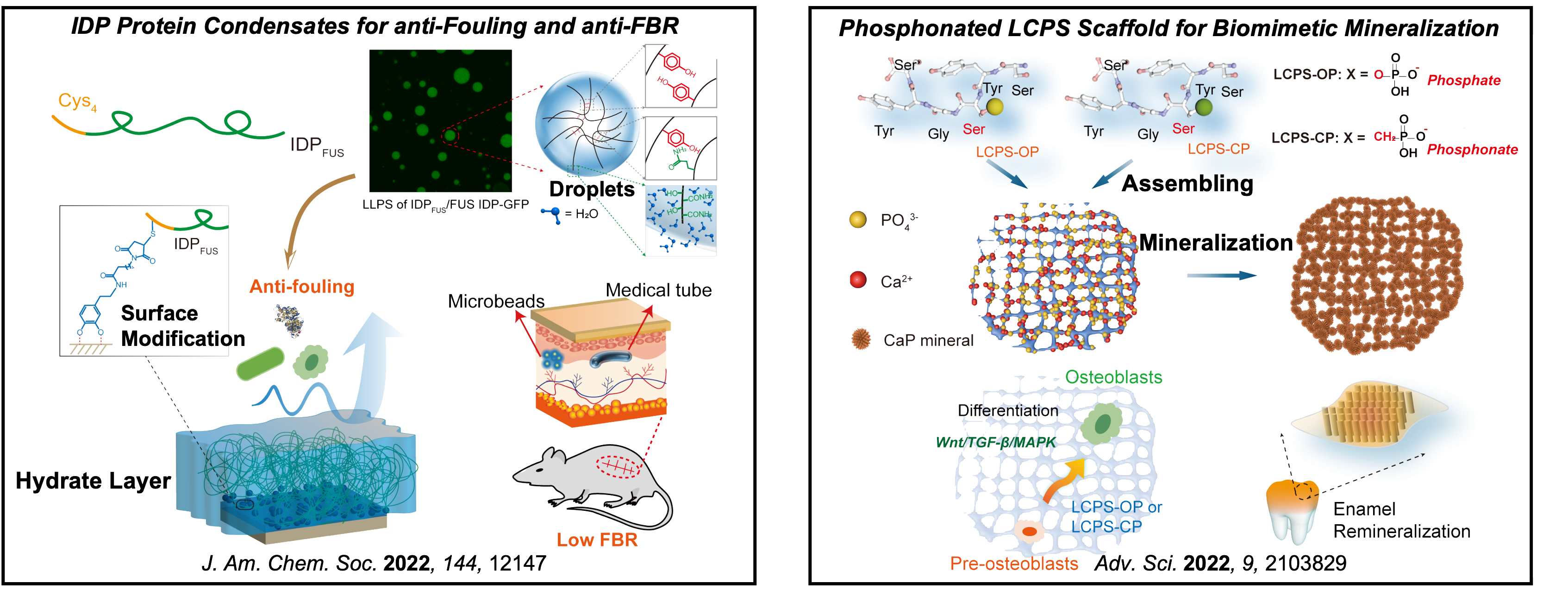
III. Modulating Bio-interfaces by Designed Peptides and Proteins for Biomedical Purpose
Owing that no therapeutics directly targeting pathological protein K-Ras in cancer and Tau in degenerative disease have been approved for clinic use, new strategies for impairing the activity of these "undruggable" but significant intracellular targets remain to be developed. We have intended to develop effective strategies for suppressing the activities of K-Ras and Tau proteins, respectively.
In addition, effective modulation of extracellular bio-interfaces requires biomimetic peptides and proteins. For example, substituents of "gold standard" poly(ethylene glycol) (PEG) are demanding for mitigation of biofouling and host's foreign body response (FBR) to implantable biomedical devices because of PEG's immunogenicity and nonbiodegradability; repair of defects in hard tissue requires biomimetic mineralized scaffolds combining cooperative effects of matrixes, additives, and cell stimulation. We have focused on developing biocompatible proteins and peptides for bio-interface modulation.